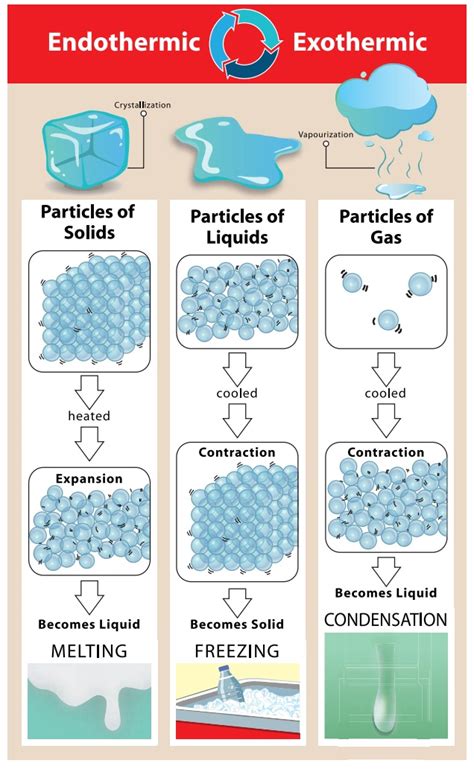
Condensation, a fundamental process in physics and chemistry, involves the transformation of a gas or vapor into a liquid state. This phenomenon is crucial in various natural and industrial processes, such as the water cycle, atmospheric science, and chemical engineering. Understanding the thermodynamic nature of condensation, specifically whether it is exothermic or endothermic, is essential for appreciating its role in energy transfer and material phase changes.
Defining Exothermic and Endothermic Processes
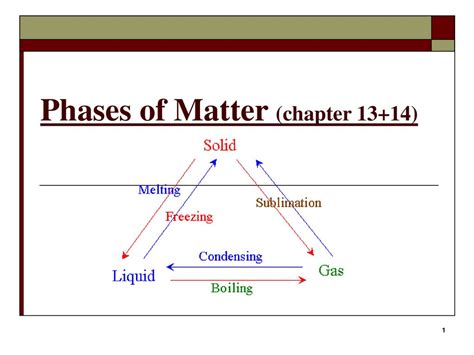
To address the question of whether condensation is exothermic or endothermic, it’s necessary to first define these terms. An exothermic process releases energy into the surroundings, typically in the form of heat, and is characterized by a negative change in enthalpy (ΔH < 0). Conversely, an endothermic process absorbs energy from the surroundings, leading to a positive change in enthalpy (ΔH > 0). These definitions are rooted in the principles of thermodynamics, which govern the relationships between heat, work, and energy.
Thermodynamic Analysis of Condensation
Condensation occurs when the temperature of a vapor decreases to its dew point, the temperature at which the vapor becomes saturated and can no longer remain in the gaseous state. During condensation, the molecules of the vapor come together, releasing potential energy as they form stronger intermolecular bonds in the liquid state. This release of energy is a hallmark of an exothermic process. For instance, when water vapor condenses into liquid water, it releases heat into the surroundings, a phenomenon that can be observed in the formation of dew or the operation of a steam engine.
| Process | Energy Change | Enthalpy Change (ΔH) |
|---|---|---|
| Condensation | Energy Release | Negative (ΔH < 0) |
| Evaporation | Energy Absorption | Positive (ΔH > 0) |
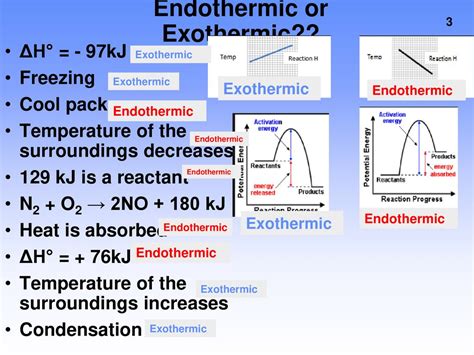
Implications and Applications

The exothermic nature of condensation has significant implications for various fields. In meteorology, the release of heat during condensation influences weather patterns and contributes to the formation of clouds and precipitation. In chemical engineering, controlling condensation conditions is critical for optimizing industrial processes, such as distillation and refrigeration. Furthermore, understanding the thermodynamics of condensation is essential for designing efficient systems for heat transfer and energy management.
Condensation in Natural Processes
In natural processes, condensation plays a vital role in the water cycle, contributing to the formation of clouds, fog, and dew. The heat released during condensation can influence local climate conditions and contribute to the Earth’s energy balance. Additionally, condensation is a key factor in the formation of precipitation, affecting agricultural productivity, water supply, and weather patterns.
Key Points
- Condensation is an exothermic process, characterized by the release of energy into the surroundings.
- The process involves the transition of a gas or vapor into a liquid state, releasing heat as molecules form stronger intermolecular bonds.
- Understanding the thermodynamic nature of condensation is crucial for appreciating its role in energy transfer and material phase changes.
- Condensation has significant implications for various fields, including meteorology, chemical engineering, and environmental science.
- The exothermic nature of condensation influences weather patterns, contributes to the formation of clouds and precipitation, and affects the design of industrial processes.
Conclusion and Future Perspectives
In conclusion, condensation is unequivocally an exothermic process, marked by the release of energy as a vapor transforms into a liquid. This understanding is foundational for a wide range of scientific and engineering applications. As research continues to refine our understanding of phase transitions and energy transfer, the importance of recognizing condensation as an exothermic process will remain a cornerstone of thermodynamic analysis and application.
What is the primary characteristic of an exothermic process?
+An exothermic process is characterized by the release of energy into the surroundings, typically in the form of heat, resulting in a negative change in enthalpy (ΔH < 0).
How does condensation affect the formation of clouds and precipitation?
+Condensation plays a critical role in the formation of clouds and precipitation by releasing heat, which influences weather patterns and contributes to the Earth’s energy balance.
What are the implications of condensation being an exothermic process for industrial applications?
+Recognizing condensation as an exothermic process is essential for optimizing industrial processes, such as distillation and refrigeration, by controlling condensation conditions to manage heat transfer and energy efficiency.