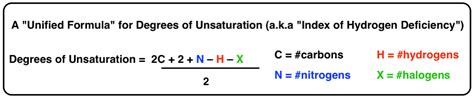
The Degrees of Unsaturation equation is a fundamental concept in organic chemistry, used to determine the number of rings and double bonds in a molecule. This equation is particularly useful for analyzing the structure of organic compounds, which can have a wide range of possible structures due to the versatility of carbon in forming bonds. The equation itself is relatively straightforward but requires a solid understanding of molecular formulas and the concept of saturation.
For any given molecule with a molecular formula of CxHyNz, where x represents the number of carbon atoms, y represents the number of hydrogen atoms, and z represents the number of nitrogen atoms, the Degrees of Unsaturation (DoU) can be calculated using the following equation: DoU = (2x + 2 + z) - y - 2z. However, a more commonly used and simplified version of this equation for hydrocarbons (compounds consisting only of carbon and hydrogen) is DoU = (2x + 2) - y, divided by 2, because each degree of unsaturation represents either a ring or a double bond, and each of these features "saves" two hydrogen atoms in the molecular formula compared to a fully saturated molecule.
Understanding the Degrees of Unsaturation Concept
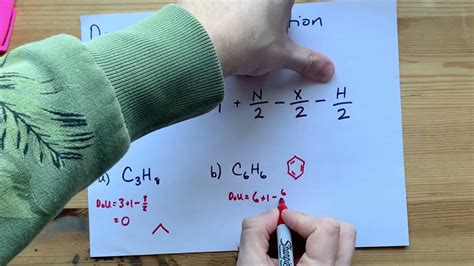
To apply the Degrees of Unsaturation equation effectively, it's crucial to understand what saturation means in the context of organic chemistry. A saturated molecule is one in which all the atoms are bonded to the maximum number of other atoms, typically with single bonds, without any multiple bonds (double or triple bonds) and without any rings. For hydrocarbons, a saturated molecule follows the general formula CxH2x+2. Any deviation from this formula, resulting in fewer hydrogen atoms, indicates the presence of either double bonds, triple bonds, or rings, which are collectively referred to as degrees of unsaturation.
Calculating Degrees of Unsaturation
Let's consider a practical example to illustrate how to calculate the Degrees of Unsaturation. Suppose we have a hydrocarbon with the molecular formula C5H8. Using the simplified equation for hydrocarbons, DoU = (2x + 2) - y, divided by 2, we substitute x = 5 (number of carbon atoms) and y = 8 (number of hydrogen atoms) into the equation. Thus, DoU = (2*5 + 2) - 8, divided by 2 = (10 + 2) - 8, divided by 2 = 12 - 8, divided by 2 = 4, divided by 2 = 2. This calculation tells us that the molecule has 2 degrees of unsaturation, meaning it could have two double bonds, one triple bond, or one ring and one double bond, among other possibilities.
| Molecular Formula | Calculation | Degrees of Unsaturation |
|---|---|---|
| C5H8 | (2*5 + 2) - 8, divided by 2 | 2 |
| C4H6 | (2*4 + 2) - 6, divided by 2 | 2 |

Applications and Limitations

The Degrees of Unsaturation equation has broad applications in organic chemistry, from identifying potential functional groups in unknown compounds to predicting the stability and reactivity of molecules based on their degree of unsaturation. However, its limitations must also be acknowledged, particularly in cases where the molecular formula does not account for other elements like oxygen, sulfur, or halogens, which can significantly affect the calculation and interpretation of degrees of unsaturation.
Key Points
- The Degrees of Unsaturation equation is a tool used to determine the number of rings and double bonds in a molecule.
- For hydrocarbons, the simplified equation DoU = (2x + 2) - y, divided by 2, is used, where x is the number of carbon atoms and y is the number of hydrogen atoms.
- The equation helps in understanding the structure of organic compounds but does not directly reveal the specific arrangement of functional groups or rings.
- Further spectroscopic analysis is required to determine the exact structure of a molecule.
- The equation has limitations, especially when dealing with molecules containing elements other than carbon and hydrogen.
In conclusion, the Degrees of Unsaturation equation is a fundamental tool in organic chemistry, offering insights into the structure of molecules. By understanding and applying this equation, chemists can better analyze and predict the properties and behaviors of organic compounds, which is crucial for advancements in fields like pharmaceuticals, materials science, and biochemistry.
What does the Degrees of Unsaturation equation tell us about a molecule?
+The Degrees of Unsaturation equation tells us the total number of rings and double bonds in a molecule, providing insights into its structure and potential reactivity.
How is the Degrees of Unsaturation calculated for hydrocarbons?
+For hydrocarbons, the Degrees of Unsaturation is calculated using the equation DoU = (2x + 2) - y, divided by 2, where x is the number of carbon atoms and y is the number of hydrogen atoms.
What are the limitations of the Degrees of Unsaturation equation?
+The equation’s limitations include its inability to account for elements other than carbon and hydrogen and its failure to reveal the specific arrangement of functional groups or rings within a molecule.