The E vs Z configuration is a fundamental concept in organic chemistry, particularly in the field of stereochemistry. It refers to the spatial arrangement of atoms or groups of atoms attached to a double bond or a ring system. Understanding the E vs Z configuration is crucial in predicting the physical and chemical properties of molecules, as well as their biological activities. In this article, we will delve into the details of the E vs Z configuration, its importance, and its applications in various fields.
Introduction to E and Z Configurations

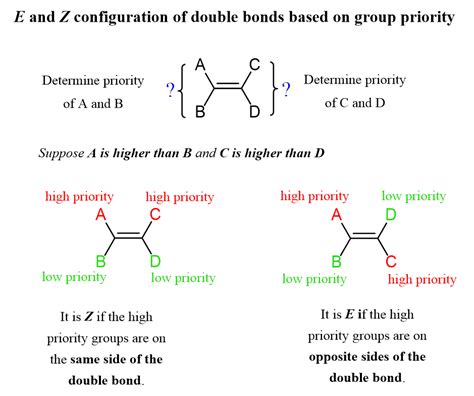
The E and Z configurations are used to describe the stereochemistry of alkenes, which are molecules containing a carbon-carbon double bond. The terms “E” and “Z” come from the German words “entgegen” and “zusammen,” meaning “opposite” and “together,” respectively. The E configuration refers to the arrangement where the two highest-priority groups are on opposite sides of the double bond, while the Z configuration refers to the arrangement where the two highest-priority groups are on the same side of the double bond.
Assigning E and Z Configurations
To assign the E or Z configuration to a molecule, we need to follow a set of rules. First, we need to prioritize the groups attached to the double bond using the Cahn-Ingold-Prelog (CIP) rules. The CIP rules state that the priority of a group is determined by its atomic number, with higher atomic numbers having higher priority. Once the priorities are assigned, we can determine the E or Z configuration by looking at the arrangement of the two highest-priority groups. If they are on opposite sides of the double bond, the configuration is E; if they are on the same side, the configuration is Z.
| Group | Priority |
|---|---|
| Hydrogen (H) | 1 |
| Carbon (C) | 2 |
| Nitrogen (N) | 3 |
| Oxygen (O) | 4 |
| Fluorine (F) | 5 |
Importance of E and Z Configurations

The E and Z configurations have significant implications in various fields, including chemistry, biology, and pharmacology. The configuration of a molecule can affect its physical and chemical properties, such as its melting point, boiling point, and reactivity. Additionally, the configuration of a molecule can influence its biological activity, with some configurations being more active than others. For example, the E configuration of a molecule may be more active than the Z configuration, or vice versa.
Applications of E and Z Configurations
The E and Z configurations have numerous applications in various fields. In chemistry, understanding the E and Z configurations is crucial in predicting the outcome of reactions, such as the addition of groups to a double bond. In biology, the E and Z configurations play a critical role in the binding of molecules to enzymes and receptors, which can affect the biological activity of the molecule. In pharmacology, the E and Z configurations can influence the efficacy and safety of drugs, with some configurations being more potent or less toxic than others.
Key Points
- The E and Z configurations describe the spatial arrangement of atoms or groups of atoms attached to a double bond or a ring system.
- The E configuration refers to the arrangement where the two highest-priority groups are on opposite sides of the double bond, while the Z configuration refers to the arrangement where the two highest-priority groups are on the same side of the double bond.
- Understanding the E and Z configurations is crucial in predicting the physical and chemical properties of molecules, as well as their biological activities.
- The E and Z configurations have numerous applications in various fields, including chemistry, biology, and pharmacology.
- The configuration of a molecule can affect its biological activity, with some configurations being more active than others.
Conclusion
In conclusion, the E vs Z configuration is a fundamental concept in organic chemistry that has significant implications in various fields. Understanding the E and Z configurations is crucial in predicting the physical and chemical properties of molecules, as well as their biological activities. The E and Z configurations have numerous applications in chemistry, biology, and pharmacology, and their importance cannot be overstated. As research continues to advance, the E and Z configurations will remain a vital part of our understanding of molecular structure and function.
What is the difference between the E and Z configurations?
+The E configuration refers to the arrangement where the two highest-priority groups are on opposite sides of the double bond, while the Z configuration refers to the arrangement where the two highest-priority groups are on the same side of the double bond.
Why are the E and Z configurations important?
+The E and Z configurations have significant implications in various fields, including chemistry, biology, and pharmacology. They can affect the physical and chemical properties of molecules, as well as their biological activities.
How are the E and Z configurations assigned?
+The E and Z configurations are assigned using the Cahn-Ingold-Prelog (CIP) rules, which prioritize the groups attached to the double bond based on their atomic number.