The realm of organic chemistry is replete with intricate mechanisms and reactive intermediates, and among these, enols and enolates hold a special place due to their crucial role in various synthetic transformations. Enols, the enol form of a carbonyl compound, and their conjugate bases, enolates, are pivotal in understanding and predicting the outcomes of numerous organic reactions, including aldol condensations, halogenations, and alkylations. This article delves into the properties, formations, and reactions of enols and enolates, highlighting their significance in organic synthesis and the underlying principles that govern their behavior.
Introduction to Enols and Enolates
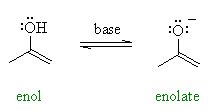
Enols are tautomeric forms of carbonyl compounds, where a hydrogen atom is bonded to a carbon that is double-bonded to an oxygen atom. This tautomeric form, although less stable than the keto form for most carbonyl compounds, is crucial for understanding the reactivity of these molecules. The conversion between the keto and enol forms is known as keto-enol tautomerism. Enolates, on the other hand, are the conjugate bases of enols, formed by the removal of a proton from the enol form, typically in the presence of a base. The formation and reactions of enolates are fundamental to many organic synthetic methods.
Formation of Enols and Enolates
The formation of enols from their keto counterparts involves the movement of a hydrogen atom and a double bond switch, a process that can be catalyzed by both acids and bases. However, the formation of enolates from enols or directly from keto forms is typically achieved through the action of a base. The choice of base can significantly influence the formation and subsequent reactions of enolates, with strong bases like sodium hydride (NaH) or lithium diisopropylamide (LDA) being commonly used for this purpose. The nature of the base, whether it is a hard or soft base, can also affect the regioselectivity of enolate formation, especially in unsymmetric ketones.
| Base | Enolate Formation Conditions |
|---|---|
| Lithium Diisopropylamide (LDA) | Tetrahydrofuran (THF), -78°C |
| Sodium Hydride (NaH) | Dimethylformamide (DMF), room temperature |

Reactions of Enols and Enolates
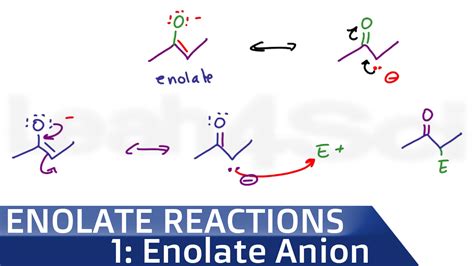
Enols and enolates are highly reactive species that participate in a wide range of organic reactions. Enols can undergo electrophilic attack at the alpha position, leading to halogenation or alkylation reactions. Enolates, being strong nucleophiles, are involved in various nucleophilic substitution and addition reactions. One of the most significant reactions of enolates is the aldol condensation, where two molecules of a carbonyl compound react to form a beta-hydroxyaldehyde or ketone, which can then undergo dehydration to yield an alpha, beta-unsaturated carbonyl compound.
Aldol Condensation
The aldol condensation is a powerful tool in organic synthesis, allowing for the formation of complex molecules from simpler precursors. This reaction involves the formation of an enolate from one molecule of the carbonyl compound, which then attacks another molecule of the carbonyl compound (or a different aldehyde) in a nucleophilic addition step, forming a beta-hydroxyaldehyde or ketone. The subsequent dehydration step can be catalyzed by either acids or bases, depending on the reaction conditions.
Key Points
- Enols and enolates are crucial intermediates in organic synthesis, participating in reactions such as aldol condensations and alkylations.
- The formation of enolates can be influenced by the choice of base, with strong bases like LDA being used for kinetically controlled enolate formation.
- Regioselectivity in enolate formation is a critical factor, especially in unsymmetric ketones, and can be influenced by the nature of the base and solvent.
- Aldol condensations provide a versatile method for forming complex molecules, including beta-hydroxyaldehydes, beta-hydroxyketones, and alpha, beta-unsaturated carbonyl compounds.
- Understanding the principles of enolate formation and reaction is essential for predicting and controlling the outcomes of these reactions in organic synthesis.
Conclusion and Future Perspectives
In conclusion, enols and enolates are fundamental to the field of organic chemistry, offering a wealth of opportunities for the synthesis of complex molecules. Their unique properties and reactivities make them invaluable tools in the hands of organic chemists. As research continues to uncover new aspects of enolate chemistry, including novel methods for enolate formation and new applications in synthesis, the importance of these intermediates will only continue to grow. For chemists aiming to push the boundaries of what is possible in organic synthesis, a deep understanding of enols and enolates is not just beneficial but essential.
What are the primary factors influencing the regioselectivity of enolate formation in unsymmetric ketones?
+The primary factors include the nature of the base (hard or soft), the solvent used, and the temperature at which the reaction is performed. These factors can influence the kinetic vs. thermodynamic control of enolate formation, leading to different regiochemical outcomes.
How does the choice of base affect the formation and reactions of enolates?
+The choice of base can significantly affect the formation and reactions of enolates. Strong bases like LDA are used for kinetically controlled enolate formation, which can lead to more regioselective reactions. The nature of the base also influences the nucleophilicity of the enolate, affecting its reactivity in subsequent reactions.
What is the significance of aldol condensations in organic synthesis?
+Aldol condensations are significant in organic synthesis because they provide a versatile method for forming complex molecules, including beta-hydroxyaldehydes, beta-hydroxyketones, and alpha, beta-unsaturated carbonyl compounds. These reactions are crucial for constructing the carbon skeletons of many natural products and pharmaceuticals.



