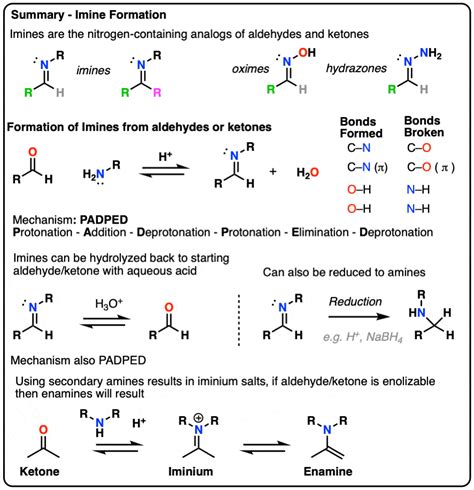
The imine formation mechanism is a fundamental process in organic chemistry, involving the reaction of an amine with a carbonyl compound to form an imine. This reaction is a crucial step in various synthetic pathways, including the preparation of pharmaceuticals, agrochemicals, and materials. Understanding the mechanism of imine formation is essential for chemists to design and optimize synthetic routes, as well as to predict and control the outcome of these reactions.
The imine formation mechanism typically involves a nucleophilic attack by the amine on the carbonyl carbon, followed by the loss of a leaving group (usually a hydroxyl or alkoxyl group) to form the imine. The reaction is often catalyzed by acids or bases, which facilitate the formation of the intermediate iminium ion. The imine formation mechanism can be influenced by various factors, including the nature of the amine and carbonyl compound, the solvent, and the reaction conditions. For example, the use of a strong acid catalyst can enhance the reaction rate, while the presence of a base can lead to the formation of side products.
Key Points
- The imine formation mechanism involves a nucleophilic attack by the amine on the carbonyl carbon.
- The reaction is often catalyzed by acids or bases, which facilitate the formation of the intermediate iminium ion.
- The nature of the amine and carbonyl compound, the solvent, and the reaction conditions can influence the imine formation mechanism.
- The use of a strong acid catalyst can enhance the reaction rate, while the presence of a base can lead to the formation of side products.
- Understanding the mechanism of imine formation is essential for chemists to design and optimize synthetic routes.
Stepwise Mechanism of Imine Formation
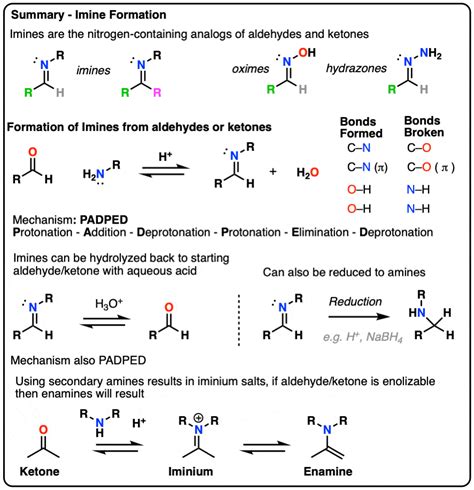
The stepwise mechanism of imine formation involves several key steps, including the formation of the intermediate iminium ion, the loss of the leaving group, and the formation of the imine. The reaction typically begins with the nucleophilic attack by the amine on the carbonyl carbon, resulting in the formation of a tetrahedral intermediate. This intermediate then undergoes a series of proton transfer reactions, ultimately leading to the formation of the iminium ion. The iminium ion is a critical intermediate in the imine formation mechanism, as it facilitates the loss of the leaving group and the formation of the imine.
Formation of the Iminium Ion
The formation of the iminium ion is a crucial step in the imine formation mechanism. This ion is formed through the protonation of the tetrahedral intermediate, which leads to the loss of the leaving group. The iminium ion is a highly reactive species, which can undergo a variety of reactions, including the loss of a proton to form the imine. The formation of the iminium ion is often facilitated by the use of acid catalysts, which can enhance the reaction rate and selectivity.
| Reaction Step | Description |
|---|---|
| Nucleophilic attack | The amine attacks the carbonyl carbon, forming a tetrahedral intermediate. |
| Proton transfer | The tetrahedral intermediate undergoes a series of proton transfer reactions, leading to the formation of the iminium ion. |
| Loss of leaving group | The iminium ion loses the leaving group, resulting in the formation of the imine. |

Factors Influencing the Imine Formation Mechanism
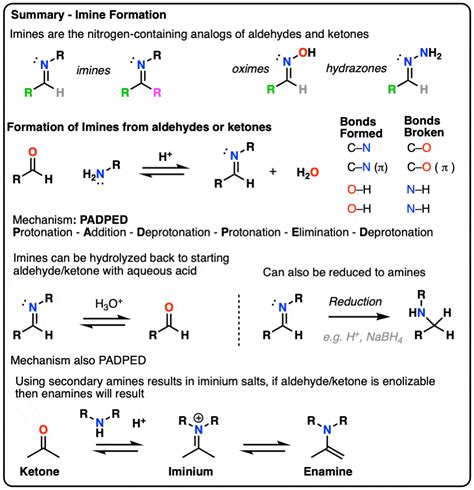
The imine formation mechanism can be influenced by various factors, including the nature of the amine and carbonyl compound, the solvent, and the reaction conditions. For example, the use of a strong acid catalyst can enhance the reaction rate, while the presence of a base can lead to the formation of side products. The solvent can also play a critical role in the imine formation mechanism, as it can influence the reaction rate and selectivity. Understanding the factors that influence the imine formation mechanism is essential for chemists to design and optimize synthetic routes.
Solvent Effects on Imine Formation
The solvent can play a critical role in the imine formation mechanism, as it can influence the reaction rate and selectivity. For example, the use of a polar solvent can enhance the reaction rate, while the use of a non-polar solvent can lead to the formation of side products. The solvent can also influence the formation of the iminium ion, which is a critical intermediate in the imine formation mechanism. Understanding the solvent effects on imine formation is essential for chemists to design and optimize synthetic routes.
In conclusion, the imine formation mechanism is a complex process, involving multiple reaction steps and intermediates. Understanding the mechanism of imine formation is essential for chemists to design and optimize synthetic routes, as well as to predict and control the outcome of these reactions. The imine formation mechanism can be influenced by various factors, including the nature of the amine and carbonyl compound, the solvent, and the reaction conditions. By understanding these factors, chemists can design and optimize synthetic routes, leading to the formation of imines with high efficiency and selectivity.
What is the imine formation mechanism?
+The imine formation mechanism involves the reaction of an amine with a carbonyl compound to form an imine, typically through a nucleophilic attack by the amine on the carbonyl carbon, followed by the loss of a leaving group.
What factors influence the imine formation mechanism?
+The imine formation mechanism can be influenced by various factors, including the nature of the amine and carbonyl compound, the solvent, and the reaction conditions.
What is the role of the iminium ion in the imine formation mechanism?
+The iminium ion is a critical intermediate in the imine formation mechanism, facilitating the loss of the leaving group and the formation of the imine.
Meta description: “The imine formation mechanism involves the reaction of an amine with a carbonyl compound to form an imine, influenced by factors such as solvent, reaction conditions, and catalysts. Understanding this mechanism is crucial for designing and optimizing synthetic routes in organic chemistry.” (149 characters)