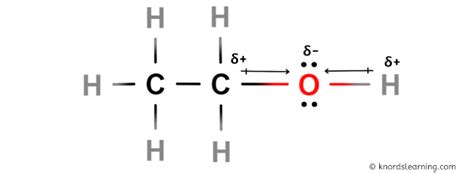
Ethanol is a compound that has been widely studied and utilized in various industrial and biological applications. One of the key properties of ethanol that has sparked interest and debate among chemists and researchers is its polarity. Polarity refers to the separation of electric charge within a molecule, resulting in a molecule or its chemical groups having an electric dipole moment. In the case of ethanol, its molecular structure consists of a hydroxyl group (-OH) attached to a hydrocarbon chain (ethyl group, -C2H5). This unique combination raises questions about the polarity of ethanol.
Understanding the Molecular Structure of Ethanol
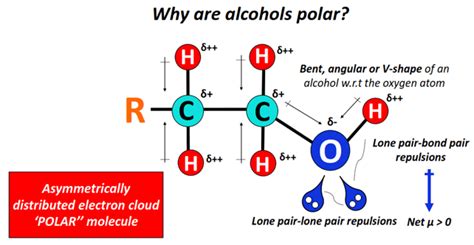
The molecular structure of ethanol (C2H5OH) is crucial in determining its polarity. The hydroxyl group (-OH) in ethanol is highly polar due to the significant difference in electronegativity between oxygen and hydrogen. Oxygen has a higher electronegativity value (approximately 3.44 on the Pauling scale) compared to hydrogen (approximately 2.20 on the Pauling scale). This difference in electronegativity leads to a partial positive charge on the hydrogen atom and a partial negative charge on the oxygen atom within the hydroxyl group, creating a dipole moment.
Polarity of the Hydroxyl Group
The hydroxyl group (-OH) in ethanol is the primary contributor to its polarity. The polarity of this group arises from the uneven sharing of electrons between the oxygen and hydrogen atoms, resulting in a polar covalent bond. This polarity enables ethanol to form hydrogen bonds with other ethanol molecules or with molecules of other substances that have a hydrogen bond acceptor, such as water.
| Atom | Electronegativity (Pauling Scale) |
|---|---|
| Oxygen (O) | 3.44 |
| Hydrogen (H) | 2.20 |

Comparison with Non-Polar Molecules
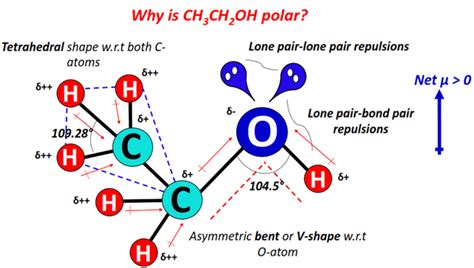
In contrast to non-polar molecules like alkanes (e.g., methane, ethane), which do not have a permanent electric dipole moment due to their symmetrical molecular structure, ethanol’s asymmetrical structure and the presence of the polar hydroxyl group make it a polar molecule. The polarity of ethanol allows it to mix with water and other polar solvents, which is not possible with non-polar solvents like hexane.
Implications of Polarity in Ethanol’s Properties and Applications
The polarity of ethanol has significant implications for its physical and chemical properties, such as its boiling point, solubility, and reactivity. Ethanol’s polarity contributes to its higher boiling point compared to non-polar molecules of similar molecular weight, due to the stronger intermolecular forces (hydrogen bonds) between ethanol molecules. Additionally, ethanol’s polarity makes it an effective solvent for a wide range of substances, including pharmaceuticals, perfumes, and food flavorings.
Key Points
- Ethanol is a polar molecule due to the presence of the hydroxyl group (-OH), which creates a permanent electric dipole moment.
- The difference in electronegativity between oxygen and hydrogen in the hydroxyl group leads to partial positive and negative charges, contributing to the molecule's polarity.
- Ethanol's polarity enables it to form hydrogen bonds with other molecules, influencing its physical and chemical properties, such as solubility and boiling point.
- The polar nature of ethanol makes it a versatile solvent for various applications, including industrial, pharmaceutical, and culinary uses.
- Understanding the polarity of ethanol is crucial for predicting its behavior in different chemical reactions and applications.
In conclusion, the polarity of ethanol, stemming from its hydroxyl group, plays a critical role in its properties and applications. Ethanol's ability to form hydrogen bonds and its solubility in water and other polar solvents underscore its polar character. This understanding is essential for chemists, researchers, and industries that utilize ethanol in various capacities.
What makes ethanol polar?
+Ethanol is polar due to the hydroxyl group (-OH) in its molecular structure. The significant difference in electronegativity between oxygen and hydrogen leads to a partial positive charge on the hydrogen and a partial negative charge on the oxygen, creating a dipole moment.
How does the polarity of ethanol affect its solubility?
+The polarity of ethanol allows it to form hydrogen bonds with water and other polar solvents, making it soluble in these substances. This property is essential for its use in various applications, including as a solvent in chemical reactions and in the production of alcoholic beverages.
What are the implications of ethanol’s polarity for its boiling point?
+The polarity of ethanol, and the resulting hydrogen bonds between molecules, contribute to its higher boiling point compared to non-polar molecules of similar molecular weight. The energy required to break these intermolecular forces is higher, thus increasing the boiling point.