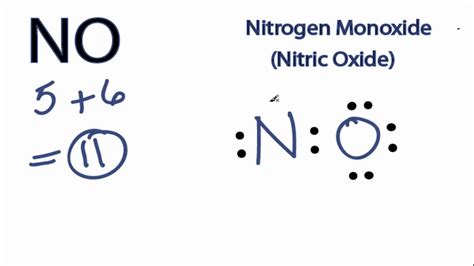
The concept of Lewis structures is a fundamental aspect of chemistry, introduced by Gilbert N. Lewis in 1916. It is a graphical representation of the distribution of electrons within a molecule, using dots to symbolize electrons and lines to represent covalent bonds. This method allows chemists to visualize the molecular structure and predict the properties of a compound. However, there are certain molecules that cannot be adequately represented by a Lewis structure, leading to the concept of "no Lewis structure" for these cases.
Limitations of Lewis Structures

Lewis structures are based on the octet rule, which states that atoms tend to gain, lose, or share electrons to achieve a full outer energy level with eight electrons. While this rule is generally applicable, there are exceptions and limitations. For instance, molecules with an odd number of electrons, such as nitrogen oxide (NO), cannot be represented by a Lewis structure that satisfies the octet rule for all atoms. Additionally, molecules with multiple bonds and those involving transition metals often pose challenges for traditional Lewis structure representation.
Odd-Electron Molecules
Odd-electron molecules, also known as free radicals, have an unpaired electron, making it impossible to satisfy the octet rule for all atoms in a traditional Lewis structure. An example of such a molecule is nitric oxide (NO), which has 11 valence electrons. Attempting to draw a Lewis structure for NO that adheres to the octet rule would result in an inaccurate representation of the molecule’s electronic configuration.
| Molecule | Number of Valence Electrons | Challenge in Lewis Structure |
|---|---|---|
| Nitric Oxide (NO) | 11 | Odd number of electrons |
| Oxygen Molecule (O2) | 12 | Multiple bonds and unpaired electrons |
| Nitrogen Dioxide (NO2) | 17 | Odd number of electrons and multiple bonds |

Molecular Orbital Theory

Molecular orbital (MO) theory provides a more comprehensive approach to understanding the electronic structure of molecules. It involves the combination of atomic orbitals to form molecular orbitals, which are a description of the distribution of electrons within the molecule. MO theory can handle molecules with odd numbers of electrons and multiple bonds more effectively than Lewis structures, offering a more accurate representation of the molecular electronic configuration.
Application of Molecular Orbital Theory
The application of MO theory can resolve the issues encountered with Lewis structures for certain molecules. For instance, in the case of the oxygen molecule (O2), MO theory can accurately predict the presence of a double bond and two unpaired electrons, which are responsible for the molecule’s paramagnetic behavior. This approach demonstrates the superiority of MO theory over traditional Lewis structures in describing the electronic configurations of complex molecules.
Key Points
- Lewis structures have limitations, particularly for molecules with an odd number of electrons or multiple bonds.
- Molecular orbital theory offers a more comprehensive approach to understanding molecular electronic structure.
- MO theory can accurately describe the electronic configuration of molecules that pose challenges for Lewis structures.
- The application of MO theory is crucial for understanding the properties and behaviors of complex molecules.
- Lewis structures should be used in conjunction with MO theory for a complete understanding of molecular chemistry.
In conclusion, while Lewis structures provide a valuable tool for understanding the electronic distribution in molecules, their limitations, particularly for odd-electron molecules and those with multiple bonds, necessitate the use of more advanced theories like molecular orbital theory. By combining these approaches, chemists can gain a deeper understanding of molecular structure and properties, ultimately contributing to advancements in various fields of chemistry and related sciences.
What are the main limitations of Lewis structures?
+The main limitations of Lewis structures include their inability to accurately represent molecules with an odd number of electrons and those with multiple bonds, as these scenarios often violate the octet rule.
How does molecular orbital theory address the limitations of Lewis structures?
+Molecular orbital theory addresses the limitations of Lewis structures by providing a more comprehensive description of the electronic structure of molecules. It can handle odd-electron molecules and multiple bonds more effectively, offering a more accurate representation of molecular electronic configurations.
What is the significance of combining Lewis structures with molecular orbital theory?
+Combining Lewis structures with molecular orbital theory is significant because it allows chemists to leverage the strengths of both methods. Lewis structures provide a simple, intuitive way to visualize molecular structure, while molecular orbital theory offers a more detailed, accurate description of electronic configurations. This combination enhances the understanding of molecular properties and behaviors.