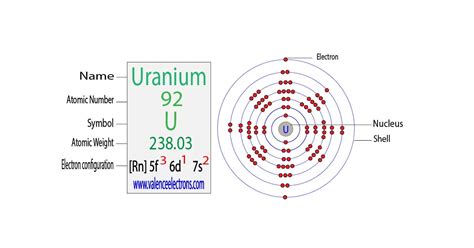
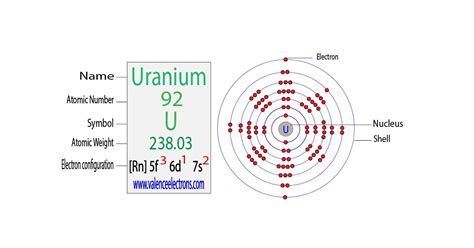
The electron configuration of an atom is a fundamental concept in chemistry and physics, describing the distribution of electrons within an atom. It is a crucial aspect of understanding the behavior of atoms, their reactivity, and the formation of chemical bonds. The electron configuration of uranium (U) is particularly complex due to its position in the periodic table as an actinide element. Uranium's atomic number is 92, which means it has 92 protons in its atomic nucleus and, in its neutral state, 92 electrons.
The electron configuration of uranium can be written in a simplified form as [Rn] 5f³ 6d¹ 7s², where [Rn] represents the noble gas core (radon core) and denotes the configuration of the inner electrons. This configuration indicates that the outermost energy levels of uranium are filled in the following way: the 5f orbital (part of the actinide series) contains three electrons, the 6d orbital contains one electron, and the 7s orbital contains two electrons. This configuration is a result of the Aufbau principle and the Hund's rule of maximum multiplicity, which dictate how electrons fill the available orbitals in an atom.
Key Points
- Uranium's atomic number is 92, influencing its electron configuration.
- The electron configuration of uranium is [Rn] 5f³ 6d¹ 7s².
- The configuration reflects the filling of electrons according to the Aufbau principle and Hund's rule.
- Understanding uranium's electron configuration is crucial for grasping its chemical properties and reactivity.
- The 5f, 6d, and 7s orbitals are involved in the outer energy levels of uranium, contributing to its unique properties.
Electron Configuration and Chemical Properties

The electron configuration of an element like uranium has a direct influence on its chemical properties. The presence of electrons in the 5f, 6d, and 7s orbitals contributes to uranium’s ability to form ions with different charges, participate in various chemical reactions, and exhibit specific physical properties. Uranium can exhibit multiple oxidation states, ranging from +3 to +6, due to the ease with which it can lose or share electrons from its outermost energy levels. This versatility in oxidation states makes uranium an interesting and complex element in terms of its chemistry.
Role of the 5f Orbitals
The 5f orbitals in uranium play a significant role in its electron configuration and, consequently, its chemical behavior. These orbitals are part of the actinide series and are involved in the bonding and reactivity of uranium compounds. The 5f electrons are considered to be inner electrons but are still relatively close to the outer surface of the atom, influencing the chemical properties of uranium. The participation of 5f electrons in bonding can lead to the formation of compounds with unique properties, such as those exhibited by uranium oxides and halides.
| Orbital | Electron Configuration | Description |
|---|---|---|
| 5f | 5f³ | Partially filled, influences chemical reactivity and bonding |
| 6d | 6d¹ | Singular electron, contributes to reactivity and oxidation states |
| 7s | 7s² | Fully filled, participates in chemical bonding and stability |
Applications and Implications

Uranium’s unique electron configuration and resulting chemical properties have significant implications for its applications. In nuclear reactors, uranium (typically as uranium-235) is used as a fuel due to its ability to undergo a controlled nuclear fission reaction. The electron configuration of uranium also plays a role in the development of new materials, including those with potential applications in energy storage, catalysis, and advanced ceramics. Understanding the electron configuration of uranium and its effects on chemical reactivity is essential for designing and optimizing these applications.
Future Perspectives
As research continues into the properties and applications of uranium and other actinides, a deeper understanding of their electron configurations will remain a critical foundation. Advances in computational chemistry and materials science will likely reveal new insights into how the electron configuration of uranium influences its behavior under various conditions. This knowledge will be vital for developing innovative technologies that safely and efficiently utilize uranium and other radioactive elements.
What is the electron configuration of uranium?
+The electron configuration of uranium is [Rn] 5f³ 6d¹ 7s², indicating how its electrons are arranged in the atom.
Why is the electron configuration important for understanding uranium's properties?
+The electron configuration influences uranium's chemical reactivity, ability to form ions, and participation in chemical reactions, making it crucial for understanding its properties and applications.
What are some applications of uranium based on its electron configuration?
+Uranium's unique electron configuration makes it useful in nuclear reactors as a fuel, in materials science for developing new materials, and in other technological applications where its chemical properties can be leveraged.
Meta Description: Understand the electron configuration of uranium and its implications for chemical properties and applications, from nuclear energy to materials science, with a deep dive into its atomic structure and reactivity.